ANDA, NDA, BLA Registration and Registration Renewal in China
ANDA: abbreviated new drug application; NDA: new drug application; BLA: biologics license application
Legal Basis of ANDA, NDA, BLA Registration and Registration Renewal
- Drug Administration Law of the People’s Republic of China (2019 Revision);
- Regulations for the Implementation of the Drug Administration Law of the People’s Republic of China (2019 Amendment);
- Measures for the Administration of Drug Registration (2020 Revision)
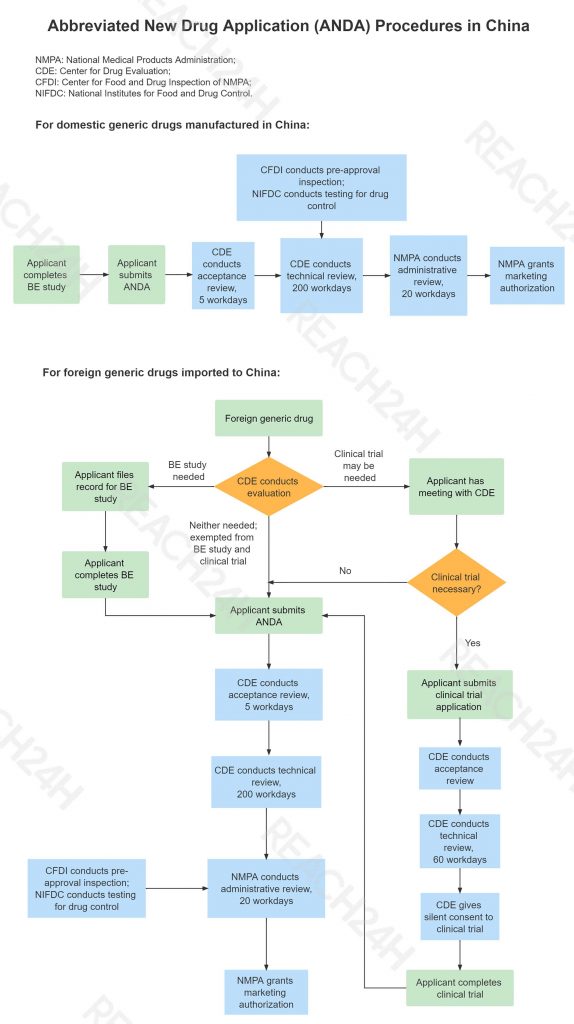
ANDA: Application for Market Approval of Generic Drugs
Product Scope
ANDA: generic drugs which are imported, sold, and used within the territory of the People’s Republic of China.
Eligibility of Applicants
Domestic applicants shall be an enterprise or drug development institution that can assume corresponding legal responsibilities. Overseas applicants shall be overseas pharmaceutical manufacturers with legal drug market approval. For overseas applicants, they must entrust a China business entity as the local agency.
Application Procedure
Domestic generic drugs, imported generic drugs
Click to see the full image of ANDA procedures.
Post-Marketing Obligation
- Change management, including major, medium, and minor-level changes and basic information change;
- Annual report.
Our Services
- Authorization to act as local agent for product filing;
- Support for application materials checklist and data gap analysis for feasibility assessment;
- Customized risk evaluation and optimized registration strategy analysis to avoid possible deficiencies in application dossier and to minimize the risk of rejection;
- Support in communications with CDE reviewers;
- Support in sample testing and NMPA on-site inspection –on-site pre-audit or mock inspection can be arranged;
- Navigate clinical trials or bioequivalence (BE) study in all stages of development, design and implementation: provide experimental design and implementation planning, assistance for Clinical Research Organization (CRO) search in China, assessment and due diligence of CROs;
- Translation of documents for non-clinical studies, including pharmacology & toxicology documents, reference journal articles etc.
- All-the-way consulting services for drug registration until approval, including communications with CDE reviewers, reply to deficiency letters from CDE, preparation and submission of supplementary applications;
- Extended service coverage after market approval, including preparation and submission of annual reports.
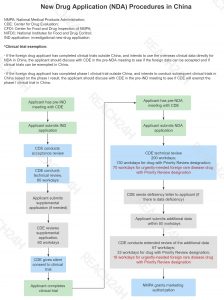
NDA: Application for Market Approval of New Drugs
Product Scope
Market approval of new drugs within the territory of the People’s Republic of China.
Eligibility of Applicants
An applicant shall be an enterprise or drug development institution that can assume corresponding legal responsibilities. Overseas applicants will must entrust a China business entity as their local agency.
Application procedure
Application for market approval of innovative new drugs and improved new drugs
Click to see the full image of NDA procedures.
Post-marketing obligation
- Change management, including major, medium, minor-level changes and basic information change;
- Annual report.
Our Services
- Feasibility assessment or data gap analysis for the NDA applications to avoid possible deficiencies in application dossier and to minimize the risk of rejection;
- Preparation of the outline of NDA application dossier;
- NDA application dossier review, translation, drafting and submission in CTD format;
- All-the-way consulting service for NDA application until approval, including communications with CDE reviewers, reply to deficiency letters from CDE, preparation and submission of supplementary applications;
- Change in application services after market approval;
- Editing and submission of annual reports.
Related Information
- China ANDA NDA: How to Get China’s Marketing Authorization for OTC Drugs
- FAQs on Drug Registration Applications & Clinical Trials | China CDE
Why Choose BaiPharm
Our experts have achieved a thorough understanding of pharmaceutical regulations and extensive experience to provide solutions across all critical stages in the drug life cycle. We’re committed to providing clients with professional and customizable pharmaceutical regulatory compliance services, including market access consultation and registration of prescription drugs, OTCs, APIs, excipients and packaging materials. Our constant driver is to ensure high-level professional and efficient solutions for pharmaceutical enterprises across the globe.