U.S. Food Contact Materials
What are food contact substances?
A Food contact substance (FCS) is any substance that can be reasonably be expected to become a component of or directly or indirectly affect the characteristics of any food. This is includes any substance intended for use in producing, manufacturing, packaging, processing, storing, transporting, or holding of food, including any source of radiation within the production process.
The following substances are not considered food contact substances:
• Substances that are generally recognized as safe (GRAS);
• A substance sanctioned prior to 1958.
Regulatory Compliance Workflow
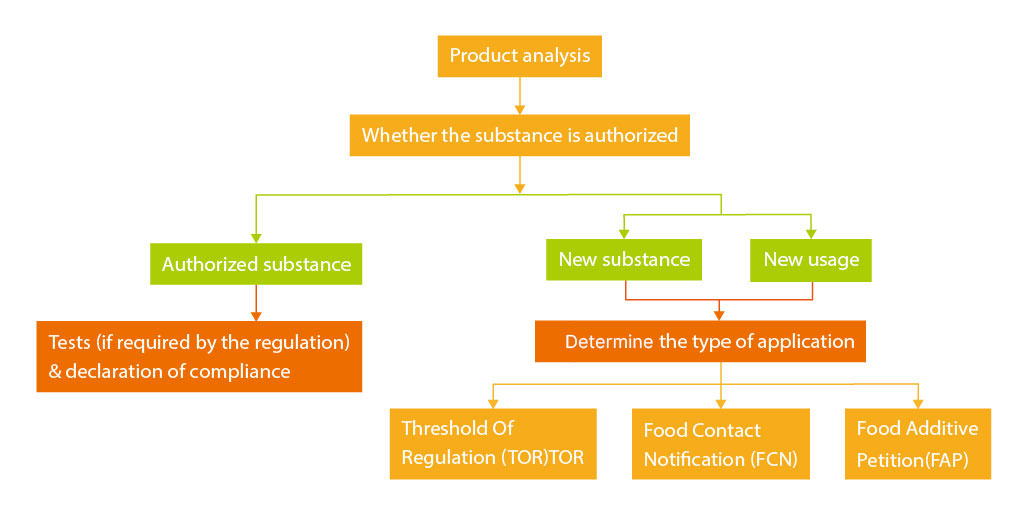
US FCS Regulatory Compliance
Declaration of Compliance
Declaration of Compliance (DoC) is a document that should be provided to downstream enterprises to ensure product safety and
regulatory compliance. Effective transmission of supply-chain information between supplier and customer helps assure a compliant status of the finished product for food contact.
Food Contact Notifications (FCN)
In order to ensure food safety, the 1958 Regulation stipulates that all food additives, both direct and indirect, need to be approved by the US
FDA before use. For indirect food additives, such as FCSs, the US FDA has established an FCN procedure that states substances that are neither approved nor forbidden for use by the US FDA for use are required to submit an FCN.
Specificity of FCN
FCN is only valid for substances producers and suppliers who submit the application. Other manufacturers / suppliers need to apply to the US FDA even though it is for the same substance.
FCN Application Workflow

Our Services
• Food contact material regulation consulting/customized consulting report
• Regulatory compliance analysis/new substance assessment
• Declaration of Compliance (DoC) editing/auditing
• Food contact notification
• Lab selection and testing supervision
• Regulatory compliance training
• Regulation translation
• Consult with competent authority
• Supplier management


