China Disinfectant Compliance
Overview
Pursuant to the “Decision of the State Council on Cancellation and Transfer of 50 Items of Administrative Permits and Other Affairs”, Decree 27 of 2013, approval of disinfectants and disinfection devices not produced by the use of new materials, new technologies, and new principles of sterilization was canceled by the National Health and Family Planning Commission (the former Ministry of Health). Under this newly deregulated market, when these disinfectants and disinfection devices are traded on the Chinese market, they are now required to implement the hygiene and safety assessment on the basis of the national hygiene standards and technical specifications. Local governments are required to conduct supervision and inspection of the enterprises and their products by law.
Regulatory System
| Issuance Date | Regulation System |
| 1-Sep-1989 | Law of China on Prevention and Control of Infectious Diseases |
| 1-July-2002
(2016 Revised) |
Ministry of Health P.R. China released Disinfection Management Measures. |
| 1-Apr-2003 | Ministry of Health P.R. China released Disinfection Technology Standards. |
| 1-May-2006 | Ministry of Health P.R. China released Label and Instruction Management Standard for Disinfectant Products. |
| 25-July-2013 | NHFPC (National Health and Family Planning Commission) released an announcement that the administrative review and approval for existing disinfectant products are no longer required. |
| 9-Dec-2013 | NHFPC (National Health and Family Planning Commission) released a Judgment basis on new materials, new process technology and new sterilization principles. |
| 11-Feb-2014 | NHFPC released an announcement on the administrative license for new materials, new processes and technologies and new sterilization principles), which is known as Three New Disinfectant Products. |
| 27-June-2014 | NHFPC released an announcement for Regulation of Hygienic Safety Evaluation for Disinfectant Products (No.36 Announcement of NHFPC〔2014〕). |
| 9-May-2017 (Revision) | Regulations on the Administration of Hygiene Administrative License for New Disinfection Products and New Aquatic Products |
Chinese National or Industry Standards
| Standard No. | Name and Content |
| WS 628 | Technical requirements for the hygiene and safety evaluation of disinfectant products |
| GB 38598 | General requirements for label and instruction of disinfection products |
| GB 38850 | List for materials and restricted substances in disinfectant |
| GB 27952 | General requirements for ordinary objects surface disinfectant |
| GB 27951 | Hygiene requirements for skin disinfectant |
| GB 27950 | General requirements for hand disinfectant |
| GB/T 27949 | General requirements of disinfectant of medical instrument |
| GB 26366 | Hygienic standard for chlorine dioxide disinfectant |
| GB 26367 | Hygienic requirements for biguanides disinfectants |
| GB 26368 | Hygienic requirements for iodine disinfectants |
| GB 26370 | Hygienic requirements for disinfectants containing bromine |
| GB 28232 | Safety and sanitation standards for ozone generator |
| GB 27955 | Hygienic requirements for low-temperature hydrogen peroxide
gas p1asma sterilizer |
| ……………. | ……………………………………………………………………………… |
Disinfectant Product Compliance
According to the “Disinfection Management Measures” and other regulations, the following tasks need to be carried out before the products (including imported disinfectants and domestic disinfectants) puts on the market:
For Fling Products
- According to GB 38598 and Judgment basis of new materials, new process technology and new sterilization principles, judge that the product does not belong to Three New Products
- According to the requirements of GB 38598-2020 “General requirement for label and instruction of disinfection products”, the label and instruction should be in compliance.
- According to the requirements of WS 628-2018 “Technical requirements for the hygiene and safety evaluation of disinfectant products”, relevant tests shall be carried out.
- According to the requirements of Regulation of Hygienic Safety Evaluation for Disinfectant Products(No.36 Announcement of NHFPC〔2014〕), the disinfection products shall be evaluated for hygiene and safety, and only products qualified in the hygiene and safety evaluation can be put on the market.
- The responsible unit of the product shall make the complete disinfection product hygiene and safety evaluation report, and submit it to the local health administration department for filling.
For New Disinfection Products
Definition
New Disinfection Products: Disinfectants and disinfection instruments are produced by using new materials, new processes and new sterilization principles.
New materials: The product that is not in the list of effective ingredients of disinfectant raw materials, not in the disinfection and antisepsis category in the Pharmacopoeia of China, and not listed in the current national hygiene standards and norms.
New processes technology: Refers to the change of production technical parameters and/or process flow, resulting in the production and processing technology of disinfectants and disinfection instruments that are equivalent to or better than conventional products in terms of effectiveness, safety and environmental adaptability.
New sterilization principles: refers to the sterilization principle and its indicator produced by physical, chemical, and biological disinfection factors or mutual synergy that are not included in the list of disinfection factors and their corresponding disinfection instruments and indicators.
Judgment basis: GB 38598 and Judgment basis of new materials, new process technology and new sterilization principles.
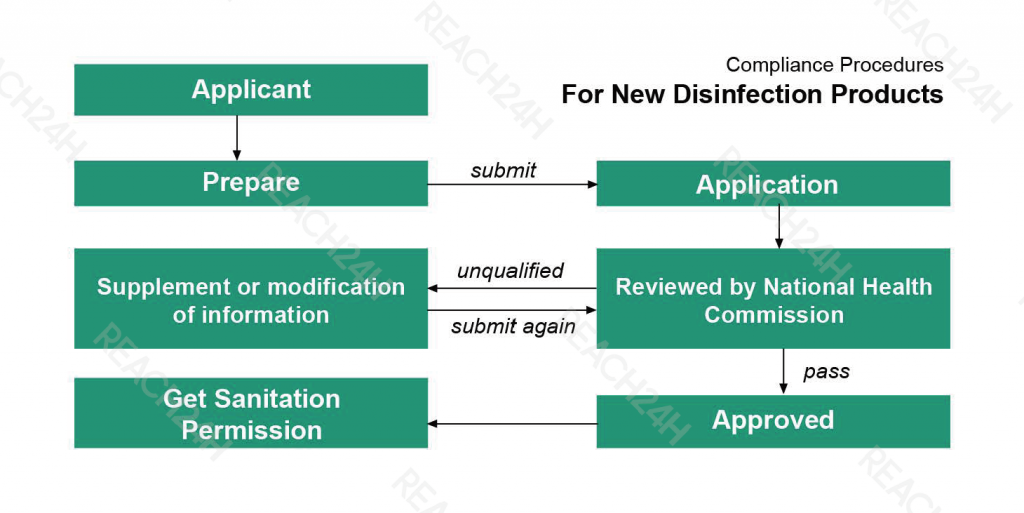
Compliance requirements: The production and import of new disinfection products shall obtain the Sanitation Permission issued by the National Health Commission (NHC).
Compliance Procedures for Disinfectant Products
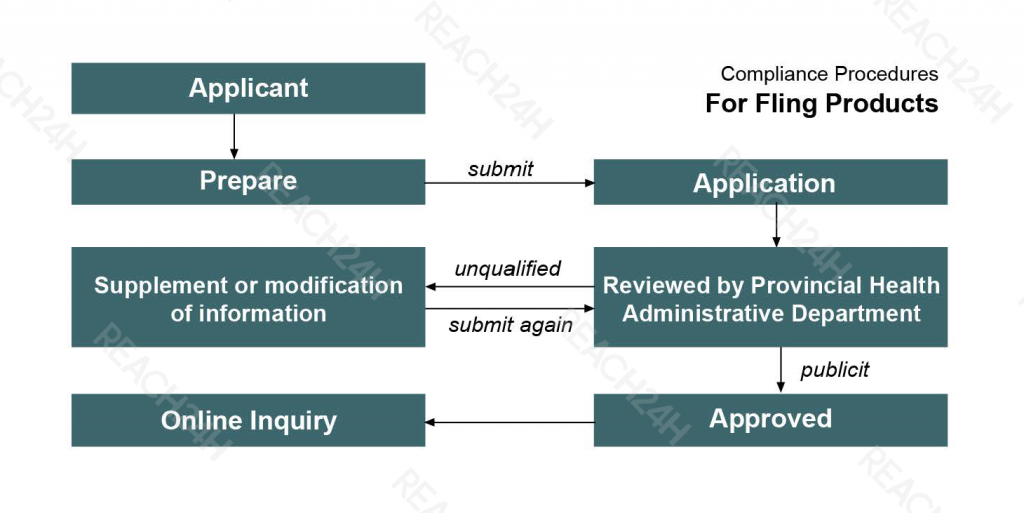
For Fling Products
Before the product sells in the market, the responsible unit of the product shall conduct the hygiene and safety evaluation, ensure the product are qualified and be responsible for the evaluation results. Then the responsible unit of the product shall prepare a complete disinfection product hygiene and safety evaluation report, and submit it to the local health administration department for filling. The filling procedures are as follows:
For New Disinfection Products:
REACH24H Chinese Disinfection Products Services
We provide comprehensive Chinese disinfectant product registration service, including
- Policy and regulation consultation
- Compliance analysis report
- Disinfection products filing
- New disinfection products declaration
- Test supervision and test report review
- Label management
- Online/On-site regulation training
Why Choose Us?
Our Strength
- Experienced Expert Team Support
- Strict Quality Control System
- Close Collaboration with Plenty of Experts and Predominant Laboratories
- Good Communication with China Authorities
- Fast Response to Clients Inquiries and Controlling Cost
- Obeying the Code of Ethics, Respecting the Confidentiality and Privacy of our Clients
- Excellent Project Management
Our Unremitting Effort in China Disinfectant Compliance Services
REACH24H has kept an eye on Disinfection Products Regulation since 2013 and followed the newly enforced regulation all the time. REACH24H can provide a one-stop service for Chinese disinfection products filing, including product type analysis, data gap analysis, compliance analysis report, trail supervision, dossier preparation and submission, etc.
REACH24H has very rich experience in Chinese disinfection product registration. REACH24H has helped the TOP 500 domestic companies as well as foreign companies in analyzing product compliance strategies, drawing up compliance plans, and finally successfully completing the China registrations.
Over the years of compliance, REACH24H has developed a good relationship and close network with testing laboratories, all these relationships would help us access the latest news of regulations, better understand regulation contents, timely know law enforcement actions from competent authorities, speed up the testing process and save testing costs.